An Element Is Made Up Of
- Pictures Of All Elements
- An Element Is Made Up Of How Many Types Of Atoms
- An Element Is Made Up Of One Type Of Atom
- An Element Is Made Up Of One Type

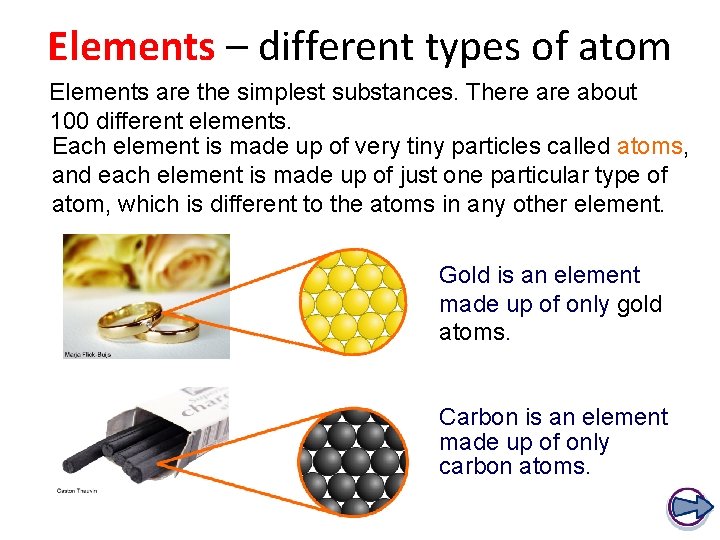
Each element is made up of just one type of atom. An atom is the smallest particle of an element that still characterizes the element. Compound A compound is a substance formed when two or more chemical elements are chemically bonded together. The type of bonds holding elements together in a compound can vary: two common types are covalent bonds and ionic bonds. Which of the following shows set of properties of an element? A Made up of big particles of atom B. Joined through chemical process Composed of one element with same properties. Joined through mechanical process. When carbon and oxygen is combined through chemical process they will end up producing a?
At 75 percent, hydrogen is the most abundant element in the universe, followed by helium at 23 percent, then oxygen at 1 percent. All of the other elements make up the remaining 1 percent. In the earth’s crust, oxygen (47%) is the most abundant element, followed by silicon (28%) and aluminum (8%). Element Names and Numbers. Favorite Answer An element is made up of three or more kinds of matter. Electrons, neutrons and protons. Although in your case the answer is false.
Elements and Atoms
An element is a pure substance. It cannot be broken down into other types of substances. Each element is made up of just one type of atom.
Structure of an Atom
An atom is the smallest particle of an element that still has the properties of that element. Every substance is composed of atoms. Atoms are extremely small, typically about a ten-billionth of a meter in diameter. However, atoms do not have well-defined boundaries, as suggested by the atomic model shown in figure (PageIndex{2}). An atom is composed of my subatomic particles. We will only discuss protons, neutron, and electrons.
| Particle | Proton | Neutron | Electron |
|---|---|---|---|
| Electric Charge | +1 | 0 | -1 |
| Location | Nucleus | Nucleus | Outside the nucleus |
| Mass | 1 amu | 1 amu | ~0 amu |
If the number of protons and electrons in an atom are equal, then an atom is electrically neutral because the positive and negative charges cancel out. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively, and it is called an ion.
The negatively charged electrons of an atom are attracted to the positively charged protons in the nucleus by a force called electromagnetic force, for which opposite charges attract. Electromagnetic force between protons in the nucleus causes these subatomic particles to repel each other because they have the same charge. However, the protons and neutrons in the nucleus are attracted to each other by a different force, called nuclear force, which is usually stronger than the electromagnetic force repelling the positively charged protons from each other.
Periodic Table of the Elements
There are almost 120 known elements. As you can see in the Periodic Table of the Elements shown in Figure (PageIndex{3}), the majority of elements are metals. Examples of metals are iron (Fe) and copper (Cu). Metals are shiny and good conductors of electricity and heat. Nonmetal elements are far fewer in number. They include hydrogen (H) and oxygen (O). They lack the properties of metals. The element most important to life is Carbon (C). Find carbon in the table. What type of element is it, metal or nonmetal?
Pictures Of All Elements

What is an element? How many elements are there?
An Element Is Made Up Of How Many Types Of Atoms
An element is a substance that is made entirely from one type of atom. For example, the element hydrogen is made from atoms containing a single proton and a single electron. If you change the number of protons an atom has, you change the type of element it is.
If you had very, very good eyes and could look at the atoms in a sample of hydrogen, you would notice that most of the hydrogen atoms would have no neutrons, some of them would have one neutron and a few of them would have two neutrons. These different versions of hydrogen are called isotopes. All isotopes of a particular element have the same number of protons, but have a different number of neutrons. If you change the number of neutrons an atom has, you make an isotope of that element.
An Element Is Made Up Of One Type Of Atom

An Element Is Made Up Of One Type
Currently, scientists know of 118 different elements. Some, like gold, silver, copper and carbon, have been known for thousands of years. Others, such as meitnerium, darmstadtium and ununquadium, have only recently been created by scientists. All known elements are arranged on a chart called the Periodic Table of Elements.
Related Pages:
For questions about this page, please contact Steve Gagnon.
